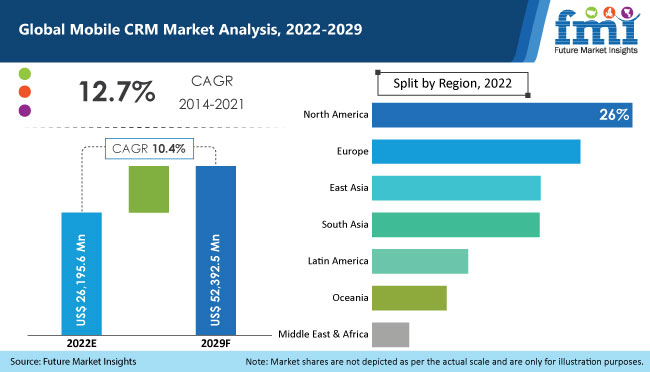
According to a new research study by Future Market Insights (FMI), the global mobile CRM market is estimated to value over US$ 26.2 Bn in 2022, up from US$ 13.5 Bn in 2021. Growth of mobile CRM market is underpinned by a slew of factors, spanning from evolving gears of technology to ubiquitous use of smartphones. Businesses, in order to adopt agility and dexterity as two of their core values, are counting on platforms that mobilize management of customer relationships. This, in turn, is likely to work in favor of mobile CRM market through 2029 and create new opportunities for the vendors to expand their offerings and reap profits.
Request a Sample of this Report @ https://www.futuremarketinsights.com/reports/sample/rep-gb-9896
As per the report, with the growth of digitized workforces, real-time access to critical information is no longer a “nice to have” aspect, rather it is a necessity to foster streamlined operations. Modern businesses are placing customer satisfaction at the top of their priority list and are actively seeking fine-grained insights for their sales realms to access on the go. This, in turn, is preparing the grounds for high scale adoption of mobile CRM platforms across the globe through 2029. According to the report, ‘CRM on demand’ is one of the overarching trends introducing major reforms in the mobile CRM market, as enterprises are increasingly turning to cloud-based suites that promise immense flexibility and scalability.
“Trends in the mobile CRM market space point to an ever-increasing end user preference for efficiency and reliability. Considering this, key vendors in the mobile CRM market space are pushing their limits and bringing out new innovations for weaving successful end user experiences”.
According to the research study, BFSI ranks among some of the early adopters of mobile CRM till 2021 and beyond, with revenues estimated to surpass US$ 3 Bn in 2022. However, retail units are likely to overtake BSFI as one of the significant spenders on mobile CRM solutions in the latter half of the assessment period, as the retailers are rapidly turning to mobile CRM platforms for streamlining the aspects of their sales funnels. Adoption of mobile CRM in healthcare is also estimated to pick pace through 2028, given that healthcare services worldwide are shifting their focus toward ‘value-based care’ models.
Ask An Analyst @ https://www.futuremarketinsights.com/ask-the-analyst/rep-gb-9896
Large enterprises are likely to lead the race of mobile CRM adoption, followed by medium enterprises, unveils the research study. As large and medium enterprises are pulling their socks up to positively engage customers and stay highly competitive, adoption of mobile CRM across these enterprises will most assuredly gain new heights. Revenue opportunities for vendors of mobile CRM market remain imminent, as sales personnel across organizations, regardless of their size, are vying to make informed decisions that are ‘data-driven’.
The pandemic of 2020 completely changed the way businesses operated under the restrictions of the pandemic. The pandemic restricted the interaction of enterprises with their customers to a certain extent and in order to keep their customers more in touch, the pandemic saw a substantial increase in the adoption of CRM solutions and systems.
Use of technology by enterprises and customers rose substantially in the pandemic era. Mobile CRM solutions saw an increase in adoption as the need for accessing customer data from anywhere in the world has increased to create customer-specific marketing solutions.
Mobile CRM solutions for businesses also saw an increase in innovations and increase in the integration of intuitive new technologies like AI and machine learning to make these CRM mobile apps more productive.
Demand for mobile-friendly CRM is anticipated to rise at a high pace in the post-pandemic era as more small businesses adopt CRM solutions to cater to their customers.
‘Pay-Per-Use’ Pricing Model: A Strategy Winning Over End Users
Considering the dynamicity of end user preferences, key vendors in the mobile CRM market are largely focused on R&D investments for unceasing developments. As price follows efficiency as the second-most important factor dictating purchase decisions, vendors are offering flexible ‘pay per use’ pricing models, as end-use preference for flexible pricing is causing a distant move away from subscription models.
Acquisitions and partnerships will continue being a vital part of the growth strategies of market players. For instance, in February 2021, AMDOCS announced its acquisition of Vubiquity, a leading firm offering premium digital content services and technology solutions. This acquisition was aimed at adding premium content capabilities, which include licensing, processing, and delivery, and positioning AMDOCS at the center of increased convergence across content community and video distributors.
Key Questions Answered in the Report
- How is demand for mobile CRM expected to progress from 2022 to 2029?
- How much is the market anticipated to be worth in 2029?
- At present, what valuation does the market for mobile CRM enjoy?
- How did the mobile customer relationship management market perform from 2014 to 2021?
- Who are the key mobile CRM solution providers?
Buy Complete Report @ https://www.futuremarketinsights.com/checkout/9896
Contact Us:
Future Market Insights
Unit No: AU-01-H Gold Tower (AU), Plot No: JLT-PH1-I3A,
Jumeirah Lakes Towers, Dubai,
United Arab Emirates
For Sales Enquiries: sales@futuremarketinsights.com
For Media Enquiries: press@futuremarketinsights.com
Website: https://www.futuremarketinsights.com