Future Market Insights (FMI) delivers key insights on the Europe viscosupplementation market in its upcoming report titled, “Viscosupplementation Market: Europe Industry Analysis and Opportunity Assessment, 2016-2026”. In terms of revenue, the Europe viscosupplementation market is projected to register a healthy CAGR of 5.1% during 2016-2026.
FMI’s report has segmented the market on the basis of product type, end user and region.
Based on product type, the market has been segmented into one injection viscosupplementation, three injection viscosupplementation and five injection viscosupplementation.
Single injection viscosupplementation product type segment is expected to emerge as the most popular segment over the forecast period, driven by better patient compliance as the treatment reduces the need for multiple hospital visits. The segment is expected to register the highest CAGR of 6.7% during the forecast period.
To remain ‘ahead’ of your competitors, request for a sample
Five injection viscosupplementation product type segment is expected to witness a decline in market share in terms of revenue from 29.0% in 2015 to 23.3% in 2026. This decline is attributed to more incidence of side effects and repeated patient visit requirements associated with five injection viscosupplementation. Reimbursement cutbacks and high injection costs are other factors that can curtail demand for five injection viscosupplementation.
By end-users, the market is segmented into hospitals, ambulatory surgical centres and orthopaedic clinics. Hospitals end-use segment is expected to witness significant demand for viscosupplementation, registering a CAGR of 4.3% over the forecast period. Better reimbursement options and increasing patient preference for single injection cycle for osteoarthritis treatment is expected to drive demand for one injection viscosupplementation in the orthopaedic clinics segment over the forecast period.
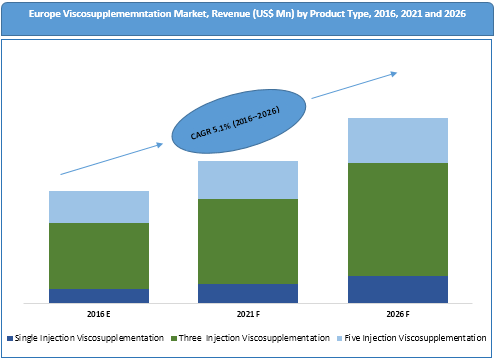
Europe Viscosupplementation Market Trends
Key trends in the viscosupplementation market are the use of single injection cycle for osteoarthritis treatment and introduction of new combination products in the market. For example, the product pipeline of major players in the viscosupplementation market involves combining corticosteroid injections, which help in relieving pain associated with osteoarthritis, with long-lasting effects of hyaluronic acid, which lasts for nearly six months. Further, regional competition within EU countries, especially in five injection cycle, is leading to entry of new market players. Europe is a mature market in terms of generic products and it is therefore relatively easier to obtain CE mark in Europe than it is to obtain the U.S. FDA approval.
Western Europe has been estimated to dominate the Europe viscosupplementation market in 2015, attributed to factors such as the presence of key regional players, strong distribution network and better healthcare infrastructure in the region.
Get a Customized Scope to Match Your Need Ask an Expert- https://www.futuremarketinsights.com/ask-question/rep-eu-1383
Key Players in Europe Viscosupplementation Market
- Anika Therapeutics Inc.
- Sanofi S.A.
- Zimmer Biomet Holdings
- Meda AB
- Ferring B.V
- Fidia Pharmaceutici S.p.A
- Bioventus LLC.
We have discussed individual strategies followed by these companies in terms of enhancing product designs, creating new manufacturing facilities, market consolidation and advanced R&D initiatives. The report concludes with key takeaways that could help players already present in the market and new players planning to enter the market in the long run.
Market Dynamics
Growth of the Europe viscosupplementation market is mainly driven by rising obesity rates and growing patient awareness about effective treatment therapies for knee osteoarthritis, coupled with macroeconomic factors such as high unmet patient needs and strengthening of distribution network by key players in the viscosupplementation market. Other prominent growth drivers include rapidly growing medical technology industry and cost-effectiveness of the treatment. However, economic issues in some Eastern European countries and reimbursement cutbacks are expected to hamper overall growth of the viscosupplementation market in Europe over the forecast period.
Viscosupplementation devices are classified under Class III category in Europe and are considered high-risk devices. They require premarket approval from notified bodies, including private organisations recognised by the European Free Trade Association (EFTA).
Key Segments
- Product Type
- Single Injection Viscosupplementation
- Three Injection Viscosupplementation
- Five Injection Viscosupplementation
- End User
- Hospitals
- Ambulatory Surgical Centres
- Orthopaedic Clinics
For critical insights, request for PDF Brochure: https://www.futuremarketinsights.com/reports/brochure/rep-eu-1383
Key Regions/Countries
- Western Europe
- U.K.
- France
- Germany
- Spain
- Italy
- Nordics
- Rest of Western Europe
- Eastern Europe
- Russia
- Rest of Eastern Europe