The global meningococcal vaccines market is expected to be driven by enhanced access to vaccines in low and middle-income countries, growing investments by manufacturers and governments to cope with pandemic meningitis outbreaks, and rapidly growing consumption of meningococcal vaccines bolstered by immunisation alliances and mass vaccination campaigns among others. Partnerships between the private sector and public institutions have been the chief agenda of global public health initiatives.
The last few years have witnessed an increase in several private-public partnerships specifically focussing on vaccine provision in developing countries. Partnerships like the International Coordinating Group on Vaccine Provision for Epidemic Meningitis Control (ICG) help magnify the vaccine outreach to remote areas of the world with the maximum need.
Request a Sample of this Report @ https://www.futuremarketinsights.com/reports/sample/rep-gb-1252
The governments of several countries across the globe initiate immunisation programmes periodically and these programmes are backed by global organisations such as UNICEF and WHO and private not for profits like the Global Alliance for Vaccines and Immunization (GAVI).
A classic example of how private-public partnerships are helping boost vaccination programmes across deep regional pockets is Brazil.
In 2010, Ezequiel Dias Foundation, Brazil formed a strategic alliance with Novartis Vaccines & Diagnostics for the sustainable supply of Menjugate MenC vaccines for Brazil’s National Immunization Program. By 2015, Brazil became self-sufficient in the production of the meningococcal C conjugated vaccine for the country’s public vaccination programmes. Such initiatives at the global level are anticipated to support global immunisation goals and at the same time boost growth of the meningococcal vaccines market.
Ask An Analyst @ https://www.futuremarketinsights.com/ask-the-analyst/rep-gb-1252
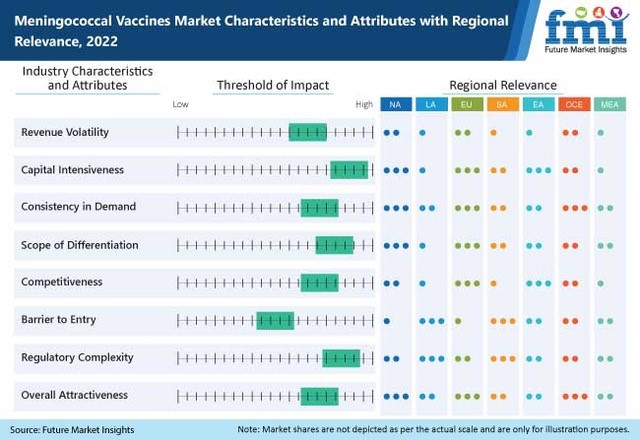
Factors Leading to Worldwide Adoption of Meningococcal Vaccination Programmes
Partnerships and alliances between manufacturers and governmental healthcare organisations are facilitating the speedy introduction of vaccines in some of the economically challenged countries of the Middle East and Africa region. Enhanced access to vaccines in under-penetrated global markets is expected to accelerate revenue growth of the global meningococcal vaccines market.
Approval of new vaccines in the U.S and Europe in 2015 and 2017 to treat meningococcal meningitis has revolutionised the global immunisation landscape. This is further boosting the growth trajectory of the global market for meningococcal vaccines.
According to Future Market Insights projections, the global market for meningococcal vaccines is expected to reach US$ 6,008.9 Mn by 2028 end from a valuation of US$ 2,290.7 Mn in 2017 – reflecting a CAGR of 9.2% during the 10 year period from 2018 to 2028.
GlaxoSmithKline to Lead the Global Meningococcal Vaccines Market with About 50% Market Share Recorded in 2017
The global meningococcal vaccines market is highly consolidated, with GlaxoSmithKline and Sanofi together holding about 80% of the global market share in 2017. Pfizer is the third largest company in this market with just under 12% market share in 2017.
Companies are expanding their production capacities through the acquisition of established vaccines production units of other global or local companies.
Sanofi has a huge pipeline of 17 active meningococcal vaccines programmes and is targeting emerging markets; while GlaxoSmithKline has 5 active pipeline programmes for meningococcal vaccines, all of which are in the phase II clinical stage. Addition of the vaccine Bexsero has enabled GlaxoSmithKline to defend its brand against Sanofi’s aggressive portfolio in the paediatric vaccines space.
- In April 2020, the U.S. Food and Drug Administration (FDA), approved Sanofi’s latest MenACWY (Meningococcal Group A,C,Y,W) conjugate vaccine “MenQuadfi” for the prevention of invasive meningococcal diseases in persons aged 2 years and older.
- In August 2020, GlaxoSmithKline plc started the patient dosing in a phase III clinical programme investigating its 5-in-1 meningitis (MenABCWY) vaccine candidate compared to licensed meningococcal vaccines, Bexsero and Menveo. The Phase 3 study is aimed at evaluating the safety, tolerability and immunogenicity of GSK’s MenABCWY vaccine candidate.
- In November 2020, European Commision (EC) approved MenQuadfi, a meningococcal conjugate vaccine for the prevention of invasive meningococcal diseases caused by Neisseria meningitidis serogroups A, C,W and Y for individuals 12 months of age and older.
Buy Complete Report @ https://www.futuremarketinsights.com/checkout/1252
Key Questions Answered in the Report
What is the meningococcal vaccines market valuation?
What is the demand outlook for meningococcal vaccines market?
At what rate did the meningococcal vaccines market grow between 2017 and 2022?
What are the key trends shaping the meningococcal vaccines market?
What will be the demand outlook for North America meningococcal vaccines market?
What is the market share of the Western Europe meningococcal vaccines market?
Who are the leading players in meningococcal vaccines market?
What is the growth projection for Japan meningococcal vaccines market?