[287 Pages Report] Having surpassed the global market valuation of US$ 1 Bn in 2021, the demand for pneumococcal testing continues to soar at a stupendous rate. A new Future Market Insights (FMI) study projects that the global pneumococcal testing market will exhibit a double-digit CAGR during the forecast period (2022 – 2030).
The use of pneumococcal testing is gathering momentum around the world owing to its current diagnostic landscape, including unmet needs and emerging technologies. Over the last decade, pneumococcal testing has undergone many technical improvements.
Increasing focus on molecular diagnostic methods for respiratory pathogen detection has yielded plethora of potential technologies. Pneumococcal testing has gained clinical significance in terms of effective ability to demonstrate high analytical sensitivity and specificity with ability to process several respiratory specimens.
Development of advanced nucleic acid detection tests and advancements in POCT products are the key factors for the growth of pneumococcal testing market over the forecast period. Moreover, rise in awareness about the benefits of early diagnosis among patient pool has increased the number of diagnostic tests in developing regions such as India, and Africa.
Additionally, increase in adoption of molecular diagnostic testing and untapped markets in the developing regions are expected to provide new avenues for the growth of pneumococcal testing market in the near future.
To remain ahead of your competitors, request for a sample – https://www.futuremarketinsights.com/reports/sample/rep-gb-11289
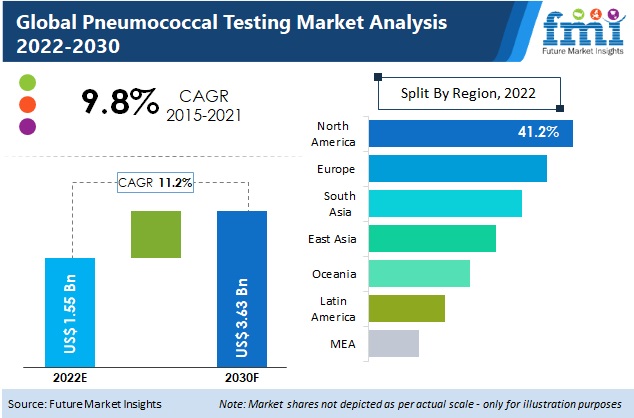
Key Takeaways of Pneumococcal Testing Market Study
- Point of care testing (POCT) remains the preferred testing methos, representing about 2/5th of total market revenue. This is attributed to rising need to provide results in real time that help physicians make informed decisions in treatment of diseases. Moreover, POCT is useful as medical care shifts to a focus on prevention, early detection, and managing acute and chronic conditions.
- Analyzers capture a leading value share in market and will maintain the trend throughout the forecast period.
- Hospitals account for over half of overall market value, given availability of pneumococcal testing and skilled personnel for better clinical management of various diseases across several medical domains.
- Enzyme linked immunosorbent assay (ELISA) accounts for major revenue share, owing to its high sensitivity and specificity, and easier to perform compared to other methods used for the detection of substances in body.
- North America and Europe collectively contribute over 70% of market share in pneumococcal testing market, with the former expected to grow at a CAGR of 12% during the forecast period.
- Increasing prevalence of pneumococcal diseases, recent advancements in pneumococcal diagnostics and government support for quality healthcare is driving the pneumococcal testing market in North America.
Diagnostic tests have the potential to improve patient care when the results are available to clinicians in a meaningful time frame. In addition, they are highly reliable to influence pathogen-directed treatment decisions, and provide important epidemiologic information.
Get a Tailored Made Report to Match Your requirements, Ask from Market Research Expert – https://www.futuremarketinsights.com/ask-question/rep-gb-11289
Tracking the COVID-19 Impact on Market Performance
The coronavirus (COVID-19) pandemic is having positive impact on market growth. The Centers for Disease Control and Prevention (CDC) has reported an upsurge in pneumonia mortalities related to the novel coronavirus.
This has spurred the adoption of molecular diagnostic tests for treating COVID-19 pneumonia. Health systems worldwide are gathering random samples from patients having severe respiratory illnesses such as pneumonia, including those with travel history, and testing them for COVID-19.
Acquisition Strategic Focus of Pneumococcal Testing Manufacturers
Acquisition strategy helps in complementing life sciences offering innovative technologies that create significant value to organizations. For example, in March 2020, Thermo Fisher Scientific Inc., the world leader in serving science acquired QIAGEN N.V., a leading global provider of molecular diagnostics and sample preparation technologies. This acquisition provides an opportunity to leverage industry-leading capabilities and R&D expertise to accelerate innovation.
FMI’s Extensive Report Coverage
Future Market Insights brings the comprehensive research report on forecasted revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2015 to 2030. The global pneumococcal testing market is segmented in detail to cover every aspect of the market and present a complete market intelligence approach to the reader.
The study provides compelling insights on pneumococcal testing market on basis of method (Immunodiagnostics, Molecular Diagnostic, Point of Care Testing), product type (Consumables, Analyzers), technology (Immunofluorescence, Enzyme Linked Immunosorbent Assay (ELISA), Western Blot Test, Nucleic Acid Sequence based Amplification, Immunohistochemistry, Polymerase Chain Reaction, Others) and end users (hospitals, ambulatory surgical centers, Clinics) across seven major regions.
For in-depth competitive analysis, Buy Now – https://www.futuremarketinsights.com/checkout/11289
Pneumococcal Testing Market by Category By Method:
- Immunodiagnostics
- Molecular Diagnostic
- Point of Care Testing
By Product:
- Consumables
- Analyzers
By Technology:
- Immunofluorescence
- Enzyme Linked Immunosorbent Assay (ELISA)
- Western Blot Test
- Nucleic Acid Sequence based Amplification
- Immunohistochemistry
- Polymerase Chain Reaction
- Others
By End User:
- Hospitals
- Ambulatory Surgical Centers
- Clinics