A comprehensive market analysis conducted in 2023 anticipates significant growth in the Atrial Appendage Occluder Industry, with projections indicating a surge from US$983.5 million in 2023 to a staggering US$8,193.0 million by 2033. This remarkable expansion is poised to be driven by a robust compound annual growth rate (CAGR) of 23.6% over the forecast period.
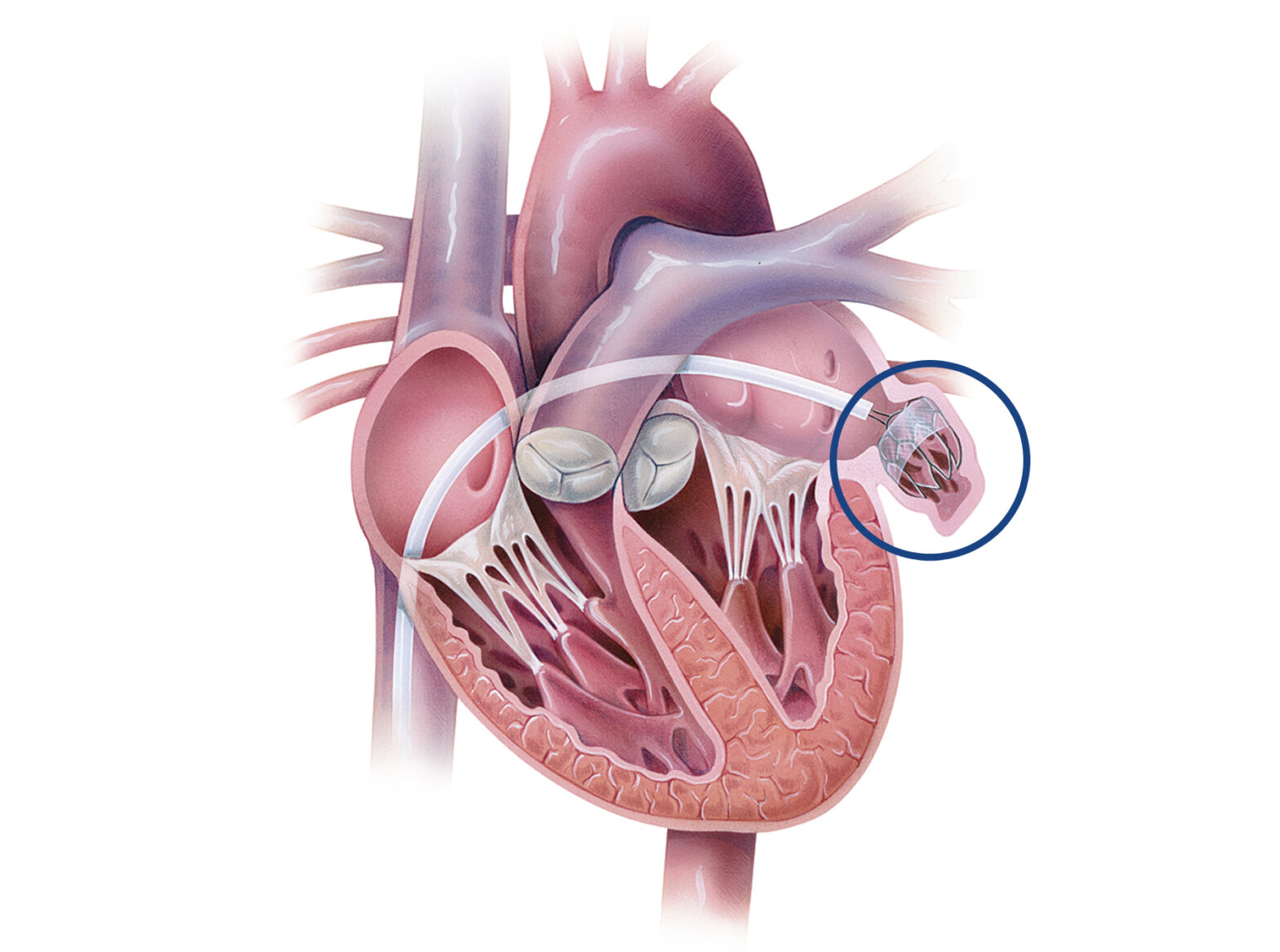
The Atrial Appendage Occluder, specifically targeting the left atrial appendage (LAA), also known as the atrial appendage, is a unique ear-shaped pouch located in the upper left chamber of the human heart, the left atrium. While its precise function remains a subject of ongoing research, it has garnered significant attention within the medical community.
Notably, individuals afflicted by arrhythmic cardiovascular diseases, such as atrial fibrillation, may experience the accumulation of blood in the LAA, potentially leading to the formation of blood clots. In the event that these blood clots enter the bloodstream, they pose a grave risk of causing life-threatening strokes.
Request a Sample Copy of This Report Now.
https://www.futuremarketinsights.com/reports/sample/rep-gb-4049
To avoid such difficulties, in addition to oral anticoagulants, an occluder can be used to permanently block off the LAA. Atrial appendage occlusion, which includes inserting a plug into the appendage, is a potential approach for protecting patients’ cardiovascular health.
According to a recent study published in the journal Clinical Medical Insights: Cardiology, atrial fibrillation affects about 2% of the global population, with the disease’s incidence expected to rise fivefold over the next four decades, particularly in the United States due to its growing elderly population.
Atrial Appendage Occluder Industry: Drivers and Restraints
The increased number of cardiovascular patients diagnosed with thromboembolism and atrial fibrillation worldwide is driving the global market for Atrial Appendage Occluders. Sedentary lifestyles and accompanying diseases such as diabetes, obesity, and hypertension have further increased demand for such devices, paving the way for market expansion.
However, the restricted usage of LAA occluders in patients at high risk of stroke and bleeding while on anticoagulant medication may limit market expansion. Furthermore, the implantation and removal of the device can result in complications such as tissue erosion, which may necessitate open-heart surgery, restricting market growth. Furthermore, unfavourable laws governing endocardial LAA closure have stifled industry expansion.
Atrial Appendage Occluder Industry: Overview
With only a few competitors, the global Atrial Appendage Occluder market is extremely consolidated. According to the World Health Organisation (WHO), over 15 million strokes occur worldwide each year. In 2010, the US spent around $53.9 billion on health care services, medications, and lost work days due to a heart attack.
Ischemic strokes, which occur when blood circulation to the brain is disrupted, account for approximately 87% of all strokes. Stroke is the third leading cause of death and the leading cause of long-term disability. Atrial fibrillation is responsible for around 20% of ischemic strokes.
Methodology Details Just a Click Away!
https://www.futuremarketinsights.com/request-report-methodology/rep-gb-4049
Atrial Appendage Occluder Industry: Region-wise Outlook
North America, Latin America, Western Europe, Eastern Europe, Asia-Pacific excluding Japan, Japan, and the Middle East and Africa are the geographical segments of the Atrial Appendage Occluder Industry. The North American market now leads the global industry, owing mostly to its vast elderly population.
Atrial Appendage Occluder Industry: Key Players
The key players in the global Atrial Appendage Occluder Industry include Appriva Medical Inc., Boston Scientific, St. Jude Medical, SentreHEART Inc, pfm medical ag., Occlutech, W. L. Gore & Associates, Inc., Cardia, Inc. and AtriCure, Inc.
Key Segments Profiled In The Atrial Appendage Occluder Industry Survey
Type of indication:
- Non-valvular Atrial Fibrillation
Procedure of positioning of the device:
- Endocardial
- Epicardial
End user:
- Hospitals
- Clinics
Level Up Your Market Understanding – Buy Now!
https://www.futuremarketinsights.com/checkout/4049
Author
Sabyasachi Ghosh (Associate Vice President at Future Market Insights, Inc.) holds over 12 years of experience in the Healthcare, Medical Devices, and Pharmaceutical industries. His curious and analytical nature helped him shape his career as a researcher.
Identifying key challenges faced by clients and devising robust, hypothesis-based solutions to empower them with strategic decision-making capabilities come naturally to him. His primary expertise lies in areas such as Market Entry and Expansion Strategy, Feasibility Studies, Competitive Intelligence, and Strategic Transformation.
Holding a degree in Microbiology, Sabyasachi has authored numerous publications and has been cited in journals, including The Journal of mHealth, ITN Online, and Spinal Surgery News.
About Future Market Insights (FMI)
Future Market Insights, Inc. (ESOMAR certified, recipient of the Stevie Award, and a member of the Greater New York Chamber of Commerce) offers profound insights into the driving factors that are boosting demand in the market. FMI stands as the leading global provider of market intelligence, advisory services, consulting, and events for the Packaging, Food and Beverage, Consumer Technology, Healthcare, Industrial, and Chemicals markets. With a vast team of over 5,000 analysts worldwide, FMI provides global, regional, and local expertise on diverse domains and industry trends across more than 110 countries.
Contact Us:
Future Market Insights Inc.
Christiana Corporate, 200 Continental Drive,
Suite 401, Newark, Delaware – 19713, USA
T: +1-845-579-5705
For Sales Enquiries: sales@futuremarketinsights.com
Website: https://www.futuremarketinsights.com
LinkedIn| Twitter| Blogs | YouTube