The Global Bone Densitometer Devices Industry is poised for exceptional growth, with market value estimates indicating a remarkable trajectory. In 2023, the market is projected to exceed US$ 347.7 million, and by 2033, it is expected to reach an impressive US$ 525.0 million, demonstrating an outstanding compound annual growth rate (CAGR) of 4.2% throughout this ten-year period.
Precision in Bone Health Assessment
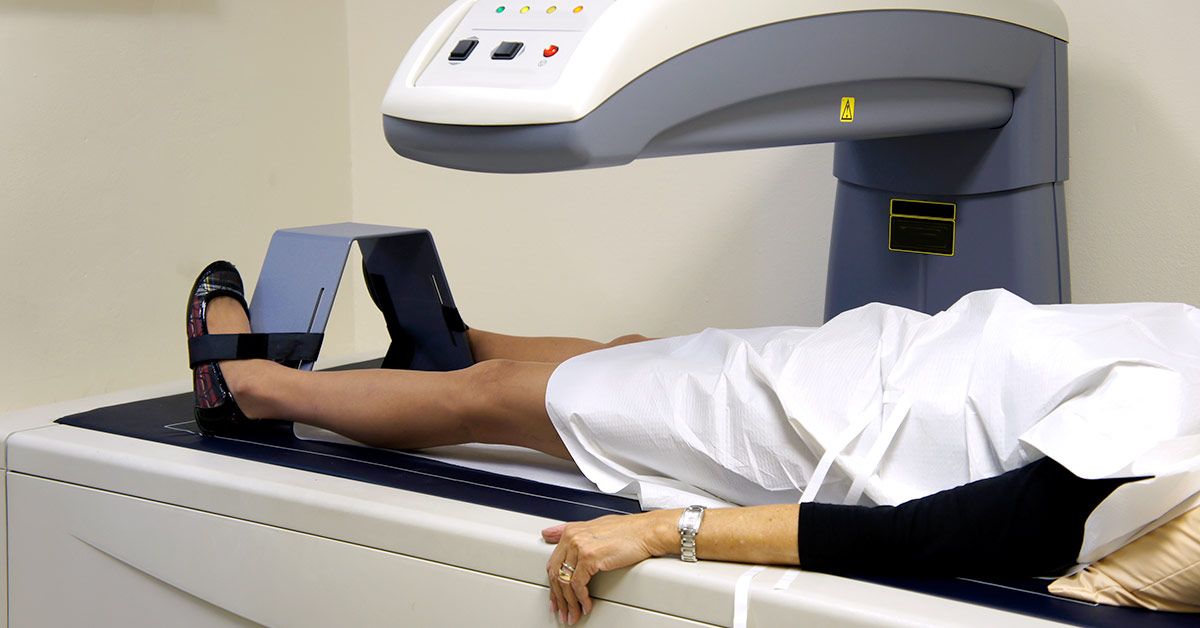
Bone densitometer devices, renowned for their non-invasive bone density assessment procedures, play a pivotal role in evaluating bone strength. These innovative devices are indispensable in diagnosing bone-related conditions and assessing the risk of their development.
A Global Health Challenge
According to the International Osteoporosis Foundation, a significant health challenge looms on the horizon. The incidence of hip fractures is predicted to rise by over 300% in men and 240% in women by 2050. Osteoporosis, along with related conditions such as osteopenia, osteomalacia, and osteoporotic fractures, is on the rise. This alarming trend underscores the pressing need for robust bone assessment, driving substantial demand for bone densitometer devices and fueling the market’s expansion.
Get a Sample Copy of the Report Now.
https://www.futuremarketinsights.com/reports/sample/rep-gb-733
Moreover, a surge in favorable reimbursement policies by Medicare will likely spur the demand for bone densitometer devices, particularly in countries like India and the U.S. Medicare provides private health insurance plans for the treatment of osteoporosis, which in turn accounts for the increasing expansion of the bone densitometer devices industry. Also, many manufacturers are keen on releasing innovative products with novel offerings like low dosage and patented narrow-fan beam technology.
This factor coupled with the rising need for excellent precision and accuracy, high-definition image quality, and faster scan for multiple clinical applications will foster an atmosphere of the growth for the target over the projected period.
“Growing bone health awareness, more widespread knowledge about osteoporosis along with initiatives from NGOs and government organizations will likely drive the market growth for the bone densitometer devices over the forecast period,” says an FMI analyst.
Key Takeaways:
- Heightened demand for precise, speedy and high quality diagnosis to strengthen the market prospects.
- High cost of the target product and limitations of the DXA technology of bone densitometer device will impede market growth.
- The U.S. will dominate the global market space with a CAGR of 4.9%.
- The market in Germany will be driven by increasing bone disorder awareness programs.
- Axial bone densitometer segment will grow at a CAGR of 4.2%
- In terms of technology, Dual Energy X-Ray Absorptiometry will hold about 3/5th of total revenue.
Methodology Details Just a Click Away!
https://www.futuremarketinsights.com/request-report-methodology/rep-gb-733
Competitive Landscape
GE Healthcare, Hologic, Inc., OSI Systems, Inc., Diagnostic Medical Systems Group, Swissray Global Healthcare Holding, Ltd., BeamMed, Ltd., Echolight S.P.A, Scanflex Healthcare AB, Medonica Co., Ltd., XinGaoYi Co., Ltd., Anjue Medical Equipment, Trivitron Healthcare, Eurotec Systems S.r.l, AMPall Co., Ltd., L’acn L’accessorio Nucleare S.R.L, Shenzhen XRAY Electric Co., Ltd., YOZMA BMTech Co., Ltd., Nanoomtech Co., Ltd., Osteosys Corporation, FURUNO Electric Co., Ltd ., and others are some of the major players in the bone densitometer devices industry profiled in the full version of the report.
Leading market players are concentrating on improving their product features to expand their business and enter new markets. Strategies like mergers, acquisitions, and distribution agreements are also employed by these enterprises to strengthen their position in the bone densitometer devices market.
Bone Densitometer Devices Industry by Category
By Product:
- Peripheral Bone Densitometer
- Axial Bone Densitometer
By Technology:
- Dual Energy X-Ray Absorptiometry (DXA)
- Peripheral Dual Energy X-Ray
- Absorptiometry (pDXA)
- Quantitative Ultrasound (QUS)
- Others
By End-User:
- Hospitals
- Orthopedic Clinics
- Diagnostic Centers
Click Here To Buy Your Full Report
https://www.futuremarketinsights.com/checkout/733
Author
Sabyasachi Ghosh (Associate Vice President at Future Market Insights, Inc.) holds over 12 years of experience in the Healthcare, Medical Devices, and Pharmaceutical industries. His curious and analytical nature helped him shape his career as a researcher.
Identifying key challenges faced by clients and devising robust, hypothesis-based solutions to empower them with strategic decision-making capabilities come naturally to him. His primary expertise lies in areas such as Market Entry and Expansion Strategy, Feasibility Studies, Competitive Intelligence, and Strategic Transformation.
Holding a degree in Microbiology, Sabyasachi has authored numerous publications and has been cited in journals, including The Journal of mHealth, ITN Online, and Spinal Surgery News.
About Future Market Insights (FMI)
Future Market Insights, Inc. (ESOMAR certified, recipient of the Stevie Award, and a member of the Greater New York Chamber of Commerce) offers profound insights into the driving factors that are boosting demand in the market. FMI stands as the leading global provider of market intelligence, advisory services, consulting, and events for the Packaging, Food and Beverage, Consumer Technology, Healthcare, Industrial, and Chemicals markets. With a vast team of over 5,000 analysts worldwide, FMI provides global, regional, and local expertise on diverse domains and industry trends across more than 110 countries.
Contact Us:
Future Market Insights Inc.
Christiana Corporate, 200 Continental Drive,
Suite 401, Newark, Delaware – 19713, USA
T: +1-845-579-5705
For Sales Enquiries: sales@futuremarketinsights.com
Website: https://www.futuremarketinsights.com
LinkedIn| Twitter| Blogs | YouTube