The global artificial pancreas device systems industry is on the brink of a transformative period, poised for an unprecedented growth trajectory. Projections indicate a staggering Compound Annual Growth Rate (CAGR) of 18.2%, steering the industry towards remarkable expansion up to 2032.
The artificial pancreas device systems industry is set to redefine the landscape of diabetes management, offering innovative solutions that cater to the needs of millions worldwide. This projected growth signifies an era of advancements in medical technology that will revolutionize how individuals manage and live with diabetes.
Get your PDF Sample Report with Latest Market Information! https://www.futuremarketinsights.com/reports/sample/rep-gb-974
The forthcoming report by Future Market Insights will leverage demand trends and growth potential to segment the market across multiple categories, geographic regions, and competitive landscapes. By examining these pivotal elements, the report aims to illuminate strategic opportunities for stakeholders and industry players navigating the Artificial Pancreas Device Systems Market.
This anticipated surge in the market signifies a substantial shift in the healthcare technology landscape, promising advanced solutions for individuals managing diabetes. The projected CAGR of 18.2% underscores the potential for innovation, investment, and impactful advancements within this critical sector.
In order to develop long-term plans and maintain company continuity in the face of crises like the continuing COVID-19 epidemic, both existing and emerging market players will benefit from the expertise contained in the Artificial Pancreas Device System market study, which is packed with critical data and forecast statistics.
To prevent the virus from spreading to people who have comorbidities or chronic conditions, doctors are delaying or postponing elective surgery unless it is absolutely required by abiding by government laws, including social distance rules and stay-at-home directions. The inability of market participants to operate as a result of movement restrictions and supply chain disruptions has led to substantial product shortages on the global market.
Key Takeaways Artificial Pancreas Device System Market:
The artificial pancreas device system (APDS) market refers to the market for devices designed to automate and optimize insulin delivery for individuals with diabetes. Here are some key takeaways regarding the APDS market:
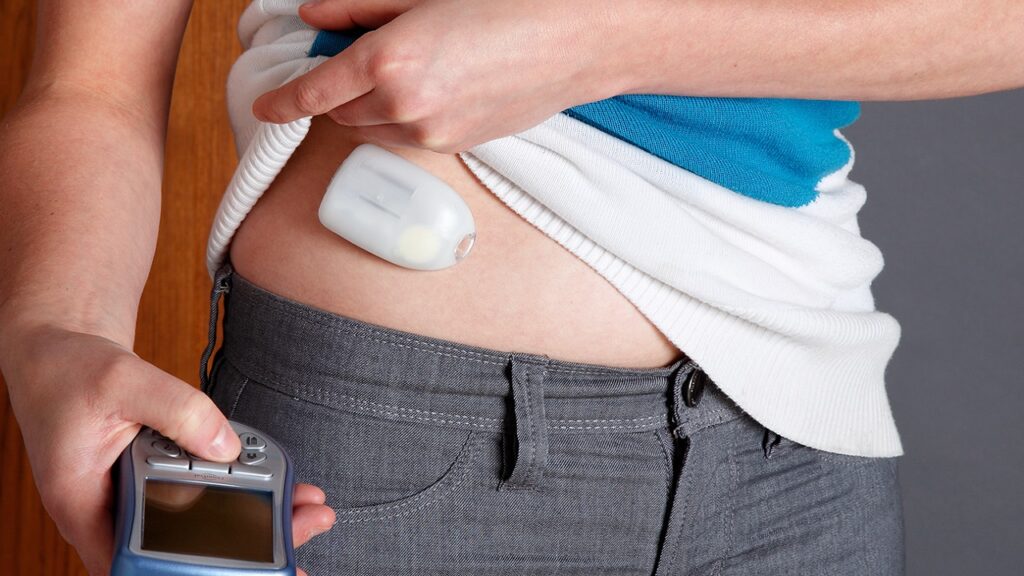
- Definition and Function: An artificial pancreas device system is a closed-loop system that combines an insulin pump, continuous glucose monitor (CGM), and a control algorithm to automatically adjust insulin delivery based on real-time glucose monitoring. Its goal is to mimic the function of a healthy pancreas by maintaining glucose levels within a target range.
- Market Growth: The APDS market has been experiencing significant growth in recent years. Factors such as the increasing prevalence of diabetes, advancements in technology, and the demand for better diabetes management solutions have contributed to the market’s expansion.
- Diabetes Prevalence: The rising prevalence of diabetes worldwide is a major driving force behind the growth of the APDS market. According to the International Diabetes Federation (IDF), approximately 463 million adults aged 20-79 had diabetes in 2019, and this number is projected to rise to 700 million by 2045.
- Improved Glucose Control: APDS offers improved glucose control compared to traditional diabetes management methods. By continuously monitoring glucose levels and adjusting insulin delivery in real-time, APDS can help prevent hypoglycemia (low blood sugar) and hyperglycemia (high blood sugar) episodes, leading to better overall diabetes management.
- Regulatory Approvals: Various APDS products have received regulatory approvals in different regions. For example, the U.S. Food and Drug Administration (FDA) approved the first hybrid closed-loop system in 2016, and subsequent systems have also gained approval. In Europe, the CE mark has been granted to several APDS devices.
Connect With Our Analyst To Address Any Inquiries You May Have! https://www.futuremarketinsights.com/ask-question/rep-gb-974
Artificial Pancreas Device System Market: Competition Analysis
The FMI’s study presents a comprehensive analysis of global, regional, and country-level players active in the Artificial Pancreas Device System market. Competitive information detailed in the Artificial Pancreas Device System market report has been based on innovative product launches, distribution channels, local networks, industrial penetration, production methods, and revenue generation of each market player. Furthermore, growth strategies and mergers & acquisitions (M&A) activities associated with the players are enclosed in the Artificial Pancreas Device System market report.
Key players covered in the report include:
- Pancreum, LLC,
- Medtronic, Inc.
- JDRF,
- Johnson & Johnson,
- Beta Bionics, Inc.,
- Tandem Diabetes Care
Important Questions Answered in the Artificial Pancreas Device System Market Report
Which end user remains the top revenue contributor in different regional markets?
- At what rate has the global Artificial Pancreas Device System market been expanding during the forecast period?
- How will the global Artificial Pancreas Device System market look like by the end of the forecast period?
- What innovative strategies are adopted by Artificial Pancreas Device System market players to stay ahead of the pack?
- What are the restraints affecting the growth of the global Artificial Pancreas Device System market?
Customization Unleashed: Learn How to Stand Out in a Competitive Landscape! https://www.futuremarketinsights.com/customization-available/rep-gb-974
Artificial Pancreas Device System Market: Segmentation
Valuable information covered in the FMI’s Artificial Pancreas Device System market report has been segregated into key segments and sub-segments.
By Device Type:
- Threshold Suspended Device System
- Control to Range (CTR) System
- Control to Target (CTT) System
By Region:
- North America
- Latin America
- Asia Pacific
- MEA
- Europe
About Future Market Insights (FMI)
Future Market Insights, Inc. (ESOMAR certified, recipient of the Stevie Award, and a member of the Greater New York Chamber of Commerce) offers profound insights into the driving factors that are boosting demand in the market. FMI stands as the leading global provider of market intelligence, advisory services, consulting, and events for the Packaging, Food and Beverage, Consumer Technology, Healthcare, Industrial, and Chemicals markets. With a vast team of over 5000 analysts worldwide, FMI provides global, regional, and local expertise on diverse domains and industry trends across more than 110 countries.
Contact Us:
Future Market Insights Inc.
Christiana Corporate, 200 Continental Drive,
Suite 401, Newark, Delaware – 19713, USA
T: +1-845-579-5705
For Sales Enquiries: sales@futuremarketinsights.com
Website: https://www.futuremarketinsights.com
LinkedIn| Twitter| Blogs | YouTube