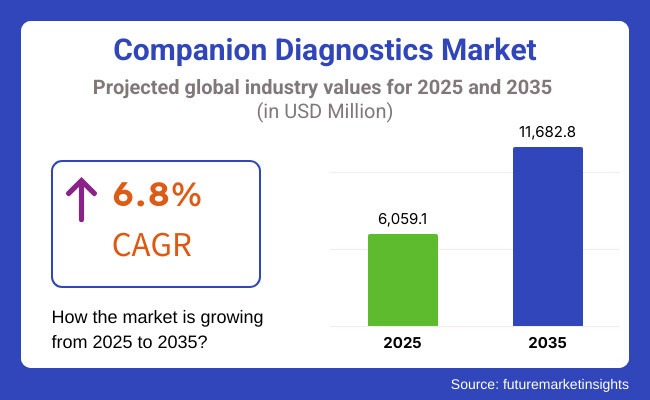
The companion diagnostics market is estimated to be valued at USD 6,059.1 million in 2025. It is projected to reach USD 11,682.8 million by 2035, registering a compound annual growth rate (CAGR) of 6.8% over the forecast period.
The companion diagnostics market has emerged as a critical component in the era of precision medicine. It plays a pivotal role in identifying patients most likely to benefit from specific treatments or therapies. By aligning diagnostic tools with therapeutic decisions, companion diagnostics help in reducing trial-and-error medication and improving patient outcomes. This market encompasses tools developed in conjunction with targeted drugs, especially in oncology, infectious diseases, and immunology. Rising demand for personalized medicine is creating a fertile landscape for market expansion.
Get Sample Report: – https://www.futuremarketinsights.com/reports/sample/rep-gb-1255
Market Trends
- Increasing partnerships between pharmaceutical and diagnostic companies is shaping the future of the companion diagnostics market.
- Integration of artificial intelligence (AI) in diagnostics is enhancing the accuracy of test results.
- Liquid biopsy-based companion diagnostics are gaining traction due to their non-invasive nature.
- The growth of next-generation sequencing (NGS) is offering faster and more comprehensive results.
- There is a shift toward multiplex diagnostics—testing for multiple markers simultaneously—for more robust data collection.
- Expansion into therapeutic areas beyond oncology, such as neurology and cardiology, marks a significant evolution.
Driving Forces Behind Market Growth
- Surge in global cancer prevalence is a major growth factor for the companion diagnostics market.
- Regulatory support and expedited approvals for targeted therapies drive innovation.
- Growing awareness among healthcare professionals about precision medicine leads to higher adoption rates.
- Technological advancements in molecular diagnostics are accelerating test development.
- Investment by key players and biotech startups is fueling research and development initiatives.
- Reimbursement frameworks are slowly evolving to support companion diagnostic testing in clinical settings.
Challenges and Opportunities
- One of the major challenges in the companion diagnostics market is the high cost associated with test development and regulatory approvals.
- Limited availability of skilled professionals to interpret complex diagnostic results hampers adoption.
- Variability in regulatory requirements across different countries leads to slower market penetration.
- However, the market also presents abundant opportunities:
- Expansion in emerging markets with improving healthcare infrastructure.
- Development of decentralized testing methods, especially in rural areas.
- Increasing integration with electronic health records (EHRs) for streamlined treatment decisions.
- Public-private partnerships in diagnostics can unlock new innovation pathways.
Regional Analysis
- North America holds the largest share in the companion diagnostics market, primarily due to strong healthcare infrastructure and early adoption of advanced technologies.
- Europe follows closely, supported by government initiatives to integrate precision medicine into standard care practices.
- Asia-Pacific is expected to witness the fastest growth owing to:
- Rising healthcare expenditure in countries like China and India.
- Increasing prevalence of chronic diseases.
- Supportive government policies to boost biotechnology research.
- Latin America and Middle East & Africa show moderate growth but offer significant potential for long-term investment.
Top Companies
- Several key players are dominating the companion diagnostics market through strategic alliances, acquisitions, and robust R&D:
- Roche Diagnostics – A pioneer in oncology-focused diagnostics with a wide product portfolio.
- Thermo Fisher Scientific – Known for its strong platform in genomic analysis.
- QIAGEN – Offers a broad range of assays and collaborates extensively with pharma companies.
- Abbott Laboratories – Continues to innovate across various disease areas.
- Agilent Technologies – Integrates precision tools for both research and clinical applications.
- BioMérieux and Myriad Genetics are also key players contributing to global market growth.
Explore In-Depth Analysis-Click Here to Access the Report:- https://www.futuremarketinsights.com/reports/companion-diagnostics-market
Segmentation Outlook
- The companion diagnostics market can be segmented based on the following categories:
- Technology:
- Polymerase chain reaction (PCR)
- Immunohistochemistry (IHC)
- Next-generation sequencing (NGS)
- In situ hybridization (ISH)
- Indication:
- Oncology (breast cancer, lung cancer, colorectal cancer)
- Cardiovascular diseases
- Neurological disorders
- Infectious diseases
- End User:
- Pharmaceutical & biotechnology companies
- Reference laboratories
- Hospitals & clinics
- Region:
- North America, Europe, Asia-Pacific, Latin America, Middle East & Africa
- Technology:
- Oncology remains the largest application segment due to the high demand for cancer-specific companion diagnostics.
- NGS-based diagnostics are expected to witness the highest CAGR due to their comprehensive genomic profiling capabilities.