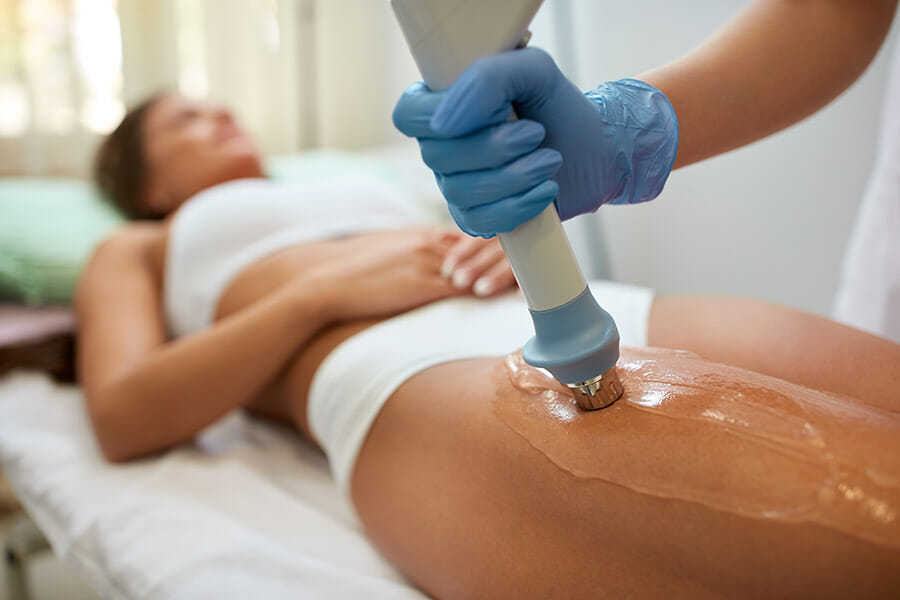
The global cellulite treatment market is expected to be valued at USD 3.67 billion in 2024 and is projected to grow at a CAGR of 7.2% during the forecast period from 2024 to 2034, eventually exceeding USD 7.37 billion by 2034.
The cellulite treatment market is gaining significant traction as awareness about skin aesthetics and body contouring continues to grow globally. Cellulite, a condition characterized by dimpled or lumpy skin, primarily affects the thighs, hips, buttocks, and abdomen. It occurs due to the underlying fat deposits pushing through the connective tissues beneath the skin. While it is a common and harmless condition, it is often considered undesirable from an aesthetic perspective, prompting many individuals to seek treatments that can help reduce its appearance.
Get Sample Report: – https://www.futuremarketinsights.com/reports/sample/rep-gb-3560
Rising beauty consciousness, social media influence, and the proliferation of cosmetic clinics have fueled demand in the cellulite treatment market. A wide range of treatment options, from non-invasive methods like laser therapy and radiofrequency to minimally invasive techniques such as subcision, are available. Moreover, innovations in topical creams and home-use devices have made treatments more accessible, contributing to market growth.
Size & Trends
The cellulite treatment market is expanding steadily, driven by increasing consumer expenditure on cosmetic procedures and wellness solutions. Changing lifestyles, sedentary habits, and rising obesity rates also contribute to the prevalence of cellulite, further enhancing demand for effective treatment options. This market is also benefiting from the rising acceptance of aesthetic procedures among both women and men, indicating a broader target demographic than in previous years.
Trends show a growing preference for non-invasive and low-downtime procedures. Consumers are leaning towards methods that promise visible results without surgical intervention or prolonged recovery periods. Additionally, social and cultural shifts toward body positivity and self-care are fueling the adoption of preventive treatments. Many consumers are investing in early-stage therapies to delay or manage the onset of cellulite, giving a new dimension to the cellulite treatment market.
Technological advancements are another significant trend in this market. New devices and formulations are being introduced regularly, enhancing both the safety and effectiveness of treatments. These innovations are not only improving clinical outcomes but also boosting consumer confidence, thereby positively influencing market dynamics.
Key Highlights
One of the most notable highlights in the cellulite treatment market is the development of advanced technologies that offer longer-lasting results with minimal discomfort. Treatments that combine multiple modalities—such as vacuum-assisted precise tissue release, laser, and radiofrequency—are gaining popularity for their comprehensive approach to cellulite reduction.
Another important highlight is the increasing number of FDA-approved treatments. Regulatory endorsements add credibility and assure safety and efficacy, which in turn increases consumer trust. Additionally, the rise of medical tourism in countries offering cost-effective cosmetic treatments has opened new avenues for market expansion.
The market has also seen an increase in awareness campaigns by dermatologists and beauty influencers who promote cellulite treatment options. Educational efforts have demystified the condition and its solutions, encouraging more individuals to explore treatment options. Clinics and brands are also leveraging digital marketing to engage and educate potential customers, enhancing market penetration.
Challenges and Opportunities
Despite its growth, the cellulite treatment market faces several challenges. High treatment costs and limited insurance coverage make these procedures inaccessible for a large segment of the population. Additionally, the varying efficacy of different treatments and lack of permanent solutions can lead to customer dissatisfaction, affecting repeat business.
Moreover, societal pressures and unrealistic beauty standards can result in skepticism and ethical debates surrounding the need for cellulite treatments. Some consumers remain hesitant, fearing side effects or doubting the effectiveness of the available solutions. These concerns can act as barriers to market adoption.
Nevertheless, the market is rife with opportunities. The increasing popularity of natural and plant-based skincare products opens new doors for topical cellulite treatments. There is also significant potential in developing personalized treatment plans based on individual skin types, severity levels, and lifestyle habits. Such approaches could significantly enhance treatment outcomes and customer satisfaction.
Expanding into untapped markets in developing countries presents another opportunity. As disposable incomes rise and beauty awareness grows in these regions, the cellulite treatment market can experience exponential growth with strategic entry and education campaigns.
Key Benefits for Stakeholders
The cellulite treatment market offers substantial benefits for a wide array of stakeholders. For consumers, the availability of diverse treatment options provides greater flexibility and access to solutions that align with their needs, comfort levels, and budgets. Many treatments today require minimal downtime, allowing users to incorporate them into their routines without significant disruption.
Clinics and dermatology centers benefit from the high-profit margins associated with cellulite treatment procedures. As demand continues to rise, businesses can enhance their service offerings and attract a broader customer base. Moreover, the integration of advanced technologies enables clinics to stay competitive and deliver superior patient outcomes.
Manufacturers and product developers also stand to gain significantly. The constant evolution in skincare and aesthetic technologies encourages innovation, enabling companies to develop products that set new benchmarks in effectiveness and safety. Pharmaceutical and cosmetic companies can diversify their product lines to cater to the expanding customer base seeking cellulite reduction solutions.
Investors and stakeholders in the health and beauty sectors can capitalize on this high-growth industry. With the right marketing strategies and product development plans, they can enjoy strong returns and long-term profitability in the cellulite treatment market.
Market Share by Geographical Region
The cellulite treatment market is geographically diverse, with notable contributions from North America, Europe, Asia-Pacific, and emerging economies in Latin America and the Middle East. North America holds a substantial share, largely due to high consumer awareness, advanced healthcare infrastructure, and strong presence of key market players. The United States, in particular, leads the region in terms of revenue generation.
Europe follows closely, with countries like Germany, France, and the UK demonstrating consistent demand for cosmetic treatments. Regulatory support for aesthetic procedures and high consumer spending on personal care drive the market in this region.
Asia-Pacific is expected to exhibit the fastest growth in the cellulite treatment market. Rapid urbanization, rising disposable incomes, and increasing awareness about beauty treatments contribute to this trend. Countries such as China, India, South Korea, and Japan are becoming lucrative markets for both international and domestic players.
In Latin America and the Middle East, the market is still developing but holds immense potential. Countries like Brazil and the UAE are witnessing growing interest in cosmetic enhancements, which could boost demand for cellulite treatment solutions in the coming years.
Competitive Outlook
The cellulite treatment market is highly competitive, with numerous players striving to capture greater market share through innovation and customer engagement. Leading companies are focusing on R&D to create more efficient and safer treatment technologies. Collaborations, partnerships, and acquisitions are common strategies used to strengthen market presence and expand product portfolios.
Some companies are investing heavily in marketing campaigns to raise awareness and build brand recognition. These efforts are especially targeted toward younger demographics and wellness-conscious consumers who are more likely to explore aesthetic enhancements. Moreover, businesses are leveraging digital platforms to offer virtual consultations and enhance customer experience, contributing to market growth.
The competitive landscape is also shaped by the entry of new players offering niche and specialized products. These companies often focus on organic or technologically unique solutions, carving out a space in the otherwise crowded market. As competition intensifies, the focus on customer satisfaction, innovation, and cost-effectiveness will define success in the cellulite treatment market.
Top Companies
Several key companies are dominating the cellulite treatment market with their innovative technologies and expansive distribution networks. These organizations include manufacturers of laser and radiofrequency devices, pharmaceutical firms developing topical treatments, and cosmetic brands that cater to at-home care.
These top companies continue to evolve their offerings to stay ahead of the competition. From launching multifunctional devices to expanding into emerging markets, they are setting the tone for the future of the cellulite treatment market. Strategic investments in clinical trials and product certifications also help these firms enhance credibility and customer trust.
Explore In-Depth Analysis-Click Here to Access the Report:- https://www.futuremarketinsights.com/reports/cellulite-treatment-market
Segmentation Outlook
The cellulite treatment market can be segmented based on treatment type, end-user, and distribution channel. Treatment types include non-invasive procedures like laser therapy, radiofrequency, and ultrasound, as well as minimally invasive techniques such as subcision and injectable treatments. Topical creams and lotions also form an essential part of the market, especially for consumers seeking at-home solutions.
In terms of end-users, the market caters to dermatology clinics, hospitals, beauty salons, and individual consumers. Clinics and salons are the dominant segments, given their access to specialized equipment and trained professionals. However, the at-home segment is growing rapidly, thanks to increasing product availability and ease of use.
Distribution channels include offline avenues like hospital pharmacies and specialty clinics, as well as online platforms that offer a wide range of products with detailed user reviews. The growing popularity of e-commerce is contributing to higher sales volumes and market visibility for various cellulite treatment solutions.
About Future Market Insights (FMI)
Future Market Insights, Inc. (ESOMAR certified, recipient of the Stevie Award, and a member of the Greater New York Chamber of Commerce) offers profound insights into the driving factors that are boosting demand in the market. FMI stands as the leading global provider of market intelligence, advisory services, consulting, and events for the Packaging, Food and Beverage, Consumer Technology, Healthcare, Industrial, and Chemicals markets. With a vast team of over 400 analysts worldwide, FMI provides global, regional, and local expertise on diverse domains and industry trends across more than 110 countries.
Contact Us:
Future Market Insights Inc.
Christiana Corporate, 200 Continental Drive,
Suite 401, Newark, Delaware – 19713, USA
T: +1-347-918-3531
For Sales Enquiries: sales@futuremarketinsights.com
Website: https://www.futuremarketinsights.com
LinkedIn| Twitter| Blogs | YouTube