The global sales of digital mobile X-ray devices is estimated to be worth USD 4,142.6 million in 2024 and anticipated to reach a value of USD 7,728.7 million by 2034. Sales are projected to rise at a CAGR of 6.6% over the forecast period between 2024 and 2034. The revenue generated by digital mobile X-ray devices in 2023 was USD 3,873.3 million. The industry is anticipated to exhibit a Y-o-Y growth of 6.1% in 2024.
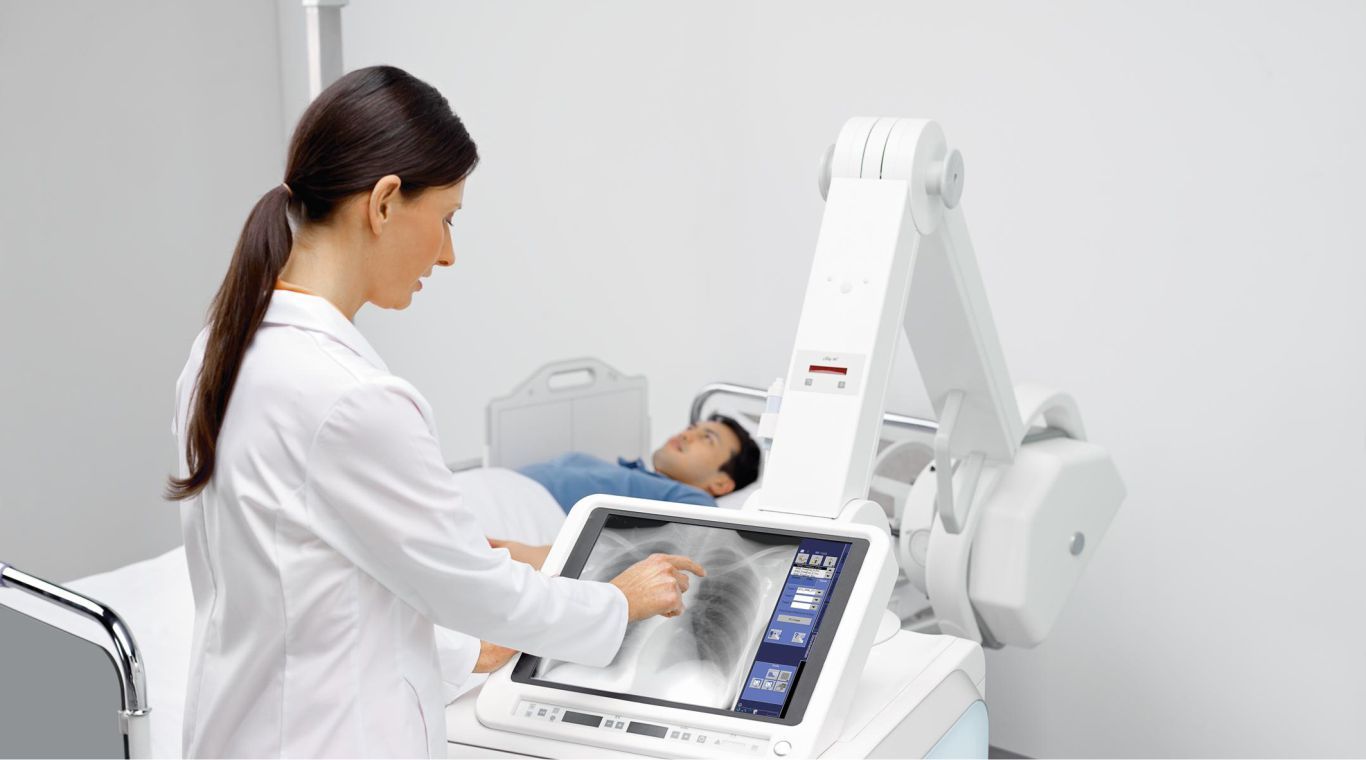
The digital mobile X-ray devices market is an important segment of the medical imaging industry that focuses on providing flexible, portable, and efficient diagnostic solutions. Digital mobile X-ray devices allow healthcare providers to perform imaging procedures outside traditional hospital settings, such as in emergency rooms, intensive care units, outpatient clinics, and remote locations. These devices integrate advanced digital technology with mobility, enhancing the convenience and speed of X-ray imaging without compromising image quality.
With increasing demand for faster diagnostics and patient-centric care, digital mobile X-ray devices are becoming essential tools in modern healthcare. They facilitate rapid clinical decision-making by delivering immediate, high-resolution images that can be easily shared and stored electronically. The growing need for point-of-care imaging, especially in critical care and trauma situations, further underscores the significance of this market. Furthermore, the reduction in radiation exposure and improved ease of use are pivotal factors driving adoption.
Get Sample Report: – https://www.futuremarketinsights.com/reports/sample/rep-gb-990
Market Trends
The digital mobile X-ray devices market is witnessing dynamic trends that reflect the broader transformation in healthcare technology. One notable trend is the adoption of wireless technology in mobile X-ray devices, enabling seamless data transfer and integration with hospital information systems. This advancement streamlines workflows, reduces delays, and enhances patient management.
Another significant trend is the miniaturization and ergonomic design of devices, making them lighter and easier to maneuver in tight or crowded environments. This enhances usability for healthcare professionals and improves patient comfort. The rise of artificial intelligence (AI) integration is also transforming this market, with AI algorithms assisting in image enhancement, anomaly detection, and automated reporting, thereby boosting diagnostic accuracy and efficiency.
There is also a growing focus on enhancing battery life and device durability to support extended use in remote or disaster-stricken areas where power availability may be limited. Additionally, regulatory approvals for digital mobile X-ray devices with improved safety features are expanding market access globally.
Challenges and Opportunities
Despite its promising growth, the digital mobile X-ray devices market faces several challenges. One major obstacle is the high initial cost of these advanced devices, which can limit adoption in small healthcare facilities and developing regions. Additionally, the requirement for trained personnel to operate and maintain digital mobile X-ray systems may constrain widespread implementation, especially in rural areas.
Data security and privacy concerns regarding the electronic transmission and storage of digital images also present challenges. Ensuring compliance with healthcare data regulations and protecting patient information are critical factors that manufacturers and providers must address.
On the opportunity side, there is significant potential for growth in emerging markets where access to advanced diagnostic equipment remains limited. Portable digital X-ray devices can revolutionize healthcare delivery in these regions by enabling early diagnosis and treatment without the need for centralized facilities. Increasing investments in healthcare infrastructure and government initiatives promoting digital health further support market expansion.
Technological innovations such as cloud-based image storage and tele-radiology services provide additional avenues for market growth. These innovations facilitate remote expert consultations and second opinions, improving diagnostic outcomes in underserved areas.
Key Regional Insights
The digital mobile X-ray devices market exhibits diverse regional characteristics shaped by healthcare infrastructure, economic development, and regulatory environments. North America holds a substantial share of the market, driven by well-established healthcare systems, high adoption rates of digital technology, and significant investments in medical imaging equipment. The United States and Canada lead the region due to advanced clinical practices and growing demand for point-of-care diagnostics.
Europe follows closely, with countries like Germany, the UK, and France showing strong market presence. The European market benefits from stringent healthcare regulations, government support for technological adoption, and an aging population that increases demand for diagnostic imaging.
The Asia-Pacific region is emerging as the fastest-growing market due to rapid urbanization, expanding healthcare infrastructure, and increasing awareness of digital health solutions. Countries such as China, India, Japan, and South Korea are key contributors, supported by rising investments in medical technology and telemedicine.
Latin America, the Middle East, and Africa represent developing markets with untapped potential. Although healthcare resources may be limited in some areas, growing efforts to improve medical services and the adoption of portable diagnostic equipment are driving market growth in these regions.
Competitive Outlook
The competitive landscape of the digital mobile X-ray devices market is characterized by intense rivalry among established multinational corporations and innovative smaller players. Market leaders focus heavily on research and development to deliver devices with superior imaging quality, enhanced mobility, and user-friendly interfaces.
Strategic partnerships, mergers, and acquisitions play a vital role in expanding product portfolios and market reach. Companies are investing in AI integration, connectivity solutions, and regulatory compliance to differentiate their offerings and meet evolving customer demands.
Customer support and service capabilities are also key competitive factors, with companies emphasizing maintenance, training, and technical assistance to build strong relationships with healthcare providers.
Emerging players that focus on niche segments, such as ultra-portable X-ray devices for field use or devices optimized for veterinary applications, contribute to the diversity and innovation within the market.
Top Companies
Several prominent companies dominate the digital mobile X-ray devices market, leveraging advanced technology and strong distribution networks. Canon Medical Systems is a major player known for its high-quality imaging solutions and commitment to innovation in mobile X-ray technology. Their devices are widely used in hospitals and clinics around the world.
Fujifilm Holdings Corporation is another key competitor, offering a broad range of digital radiography products with a focus on image clarity, portability, and integrated workflow solutions. Their continuous investment in R&D supports new product launches and market expansion.
Siemens Healthineers is recognized for its cutting-edge medical imaging equipment, including mobile digital X-ray devices that combine precision imaging with ergonomic design. Their global presence and comprehensive customer service contribute to their strong market position.
Other significant players include Carestream Health, Konica Minolta, and Agfa-Gevaert, each bringing unique strengths in digital imaging technology and healthcare solutions. These companies emphasize product innovation, regulatory compliance, and strategic collaborations to maintain competitiveness.
Explore In-Depth Analysis-Click Here to Access the Report:- https://www.futuremarketinsights.com/reports/digital-mobile-x-ray-market
Segmentation Outlook
The digital mobile X-ray devices market can be segmented based on product type, application, end-user, and technology. In terms of product type, handheld digital mobile X-ray devices and cart-based systems are the primary categories. Handheld devices offer extreme portability and convenience for point-of-care diagnostics, while cart-based systems typically provide enhanced imaging capabilities and support for multiple clinical settings.
Applications include general radiography, orthopedics, emergency care, pulmonology, and others. Emergency care and critical care settings represent significant market segments due to the need for rapid and accurate imaging in trauma cases.
End-users comprise hospitals, diagnostic centers, outpatient clinics, and field medical services. Hospitals remain the largest consumers, but the demand from outpatient and remote care facilities is growing rapidly as healthcare models shift towards decentralized services.
Technological segmentation covers wireless and wired digital mobile X-ray devices. Wireless devices, with their ease of connectivity and flexibility, are gaining preference among healthcare providers, while wired devices continue to serve settings that require stable, uninterrupted imaging capabilities.
About Future Market Insights (FMI)
Future Market Insights, Inc. (ESOMAR certified, recipient of the Stevie Award, and a member of the Greater New York Chamber of Commerce) offers profound insights into the driving factors that are boosting demand in the market. FMI stands as the leading global provider of market intelligence, advisory services, consulting, and events for the Packaging, Food and Beverage, Consumer Technology, Healthcare, Industrial, and Chemicals markets. With a vast team of over 400 analysts worldwide, FMI provides global, regional, and local expertise on diverse domains and industry trends across more than 110 countries.
Contact Us:
Future Market Insights Inc.
Christiana Corporate, 200 Continental Drive,
Suite 401, Newark, Delaware – 19713, USA
T: +1-347-918-3531
For Sales Enquiries: sales@futuremarketinsights.com
Website: https://www.futuremarketinsights.com
LinkedIn| Twitter| Blogs | YouTube